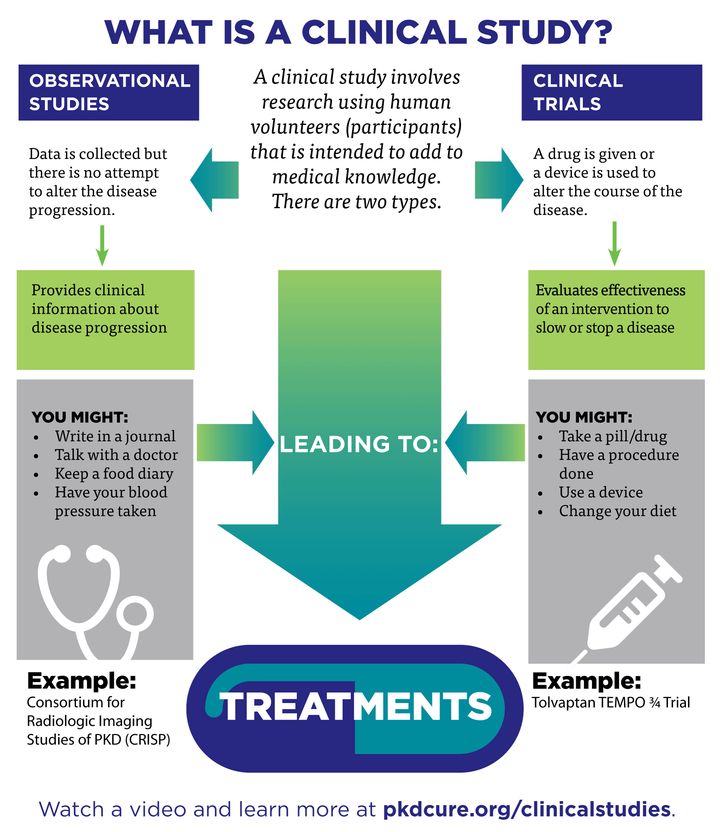
Only one-third of trials at US medical centers get reported within two years of completion.
Just last month, Daniel Cressey and the science journal Nature released a study about the challenges facing medical centers and physicians as they attempt to make use of clinical trial data.
This is directly related to the Clinical Trials Innovation Prize. As mentioned in an earlier HeroX article, lack of participation has a drastic impact on the completion of trials. It's easy to draw a connection to the problems described by Cressey in Nature. If one out of five clinical trials is not completed because of insufficient engagement, this likely also negatively impacts the ability of researchers to report their findings in a timely matter.
Imagine: A medical center manages to obtain enough participants to complete its research, great! However, It spent too much time or money finding trial participants to complete the actual research itself.
This is simply ridiculous. According to the Department of Health and Human Services, an average clinical trial costs over 30 million dollars. That is a serious chunk of cash (not to mention the time and effort of professionals and volunteers) to result in an "inconclusive" status because of a lack of participants, then even further depreciated by a delayed report.
“The lack of timely reporting and publication fundamentally impairs the research enterprise, violates the commitment made by investigators to patients and funders, squanders precious time and resources, and threatens to compromise evidence-based clinical decision making,” says the research at Yale University in New Haven, Connecticut.
This tragic lack of effectiveness in something, so crucial to the progress of medical science, is hard to hear about -- especially for those among us who have lost a loved one to disease. Regardless of the current reality, there's still hope for improvement. We can innovate our way out of these repeat issues; most especially lacking participation.
What great solution will bring the positive tipping point for participation in Clinical trials?
Throw your hat (and hopefully great idea) into the ring here, by signing up for the Clinical Trials Innovation Prize today.