Introduction
Each year, in honor of World Cancer Day, the Bonnie J. Addario Lung Cancer Foundation (ALCF) launches a unique, international crowdsourcing challenge that leverages the power of the crowd to solve problems plaguing the oncology clinical medicine space.
We want to work with innovative, out-of-the-box thinkers and innovators who are passionate about changing the lives of thousands of people- cancer patients today, and patients of tomorrow- regardless of whether they have any direct healthcare experience.
For 2016 World Cancer Day, we want YOU to join us and #BeTheSolution!
The What?
On February 4, 2016 are launching the second phase of the Clinical Trial Innovation Prize, an international two-part, $180,000 crowdsourcing challenge that seeks to identify innovative ways to increase cancer patient enrollment in clinical trials.
Goal: The goal of the challenge is to produce breakthroughs that will increase the patient accrual rate to oncology clinical trials. ALCF is specifically looking to:
- Identify prototypes of solutions that reduce a barrier to participation in clinical trials
- Select the best solution to be scaled in partnership with the Bonnie J. Addario Lung Cancer Foundation
- Ideation Challenge: 47 Competitors from 18 countries from all around the world submitted creative and novel ideas to double the accrual rate of cancer clinical trials, and three teams won prizes totaling $30,000 USD. Congratulations to Helynx, Matt Gerber, and Noah Craft, the winners of the first phase of the challenge!
- Implementation/Proof of Concept Challenge: Register now, enter your idea and plans for implementation for a chance to win prizes totaling $150,000 USD when you provide proof that your ideas have indeed resulted in an increase in participation in cancer clinical trials.
The Why?
Cancer is one of the leading causes of deaths worldwide! Cancer cases worldwide are forecast to rise by 75% and reach close to 25 million over next two decades


We need better ways to prevent, detect, screen and treat cancer, and we need them now!
The most reliable and the only accepted scientific method to take exciting discoveries from the lab bench to the bedside is testing the safety and efficacy of these new interventions in clinical trials -- research studies that test new methods of prevention, detection, screening and treatment of cancer.
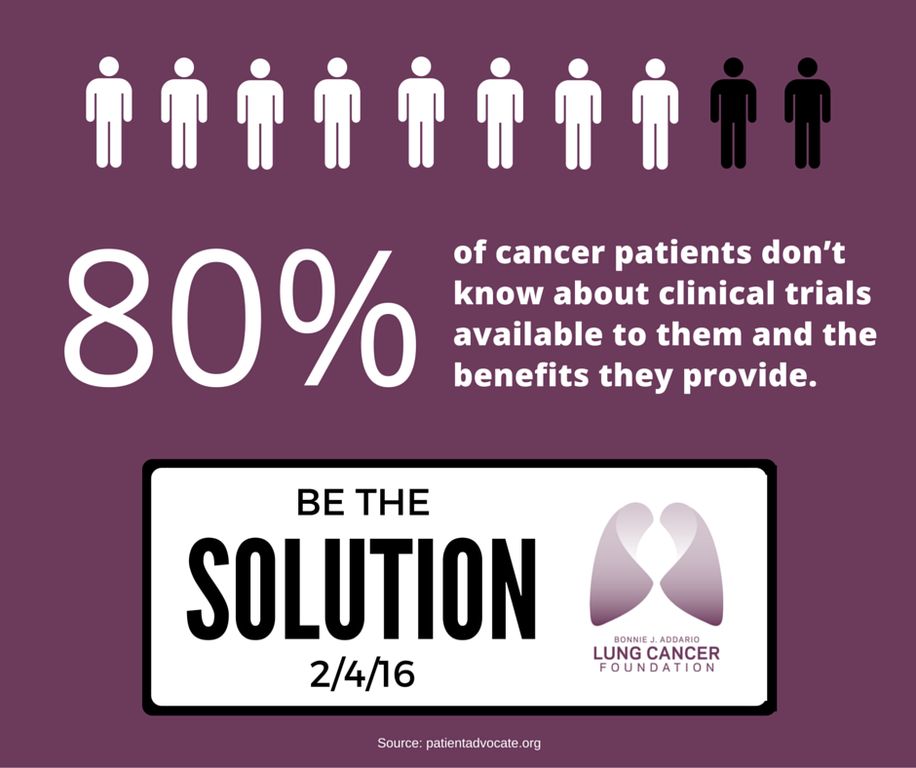
Unfortunately, most cancer patients don't know that clinical trials may be a treatment option, and there are several myths and misconceptions associated with clinical trials!

Due to a variety of factors such as myths and misconceptions; geographic, language and socio-economic barriers; lack of awareness among patients and physicians; procedural inefficiences (such as complexities in enrolling in the trial itself, the informed consent process), etc., several trials are halted prematurely as they FAIL to enroll enough patients, thereby wasting precious research dollars and impeding the development of life-saving cancer diagnostics and treatments.
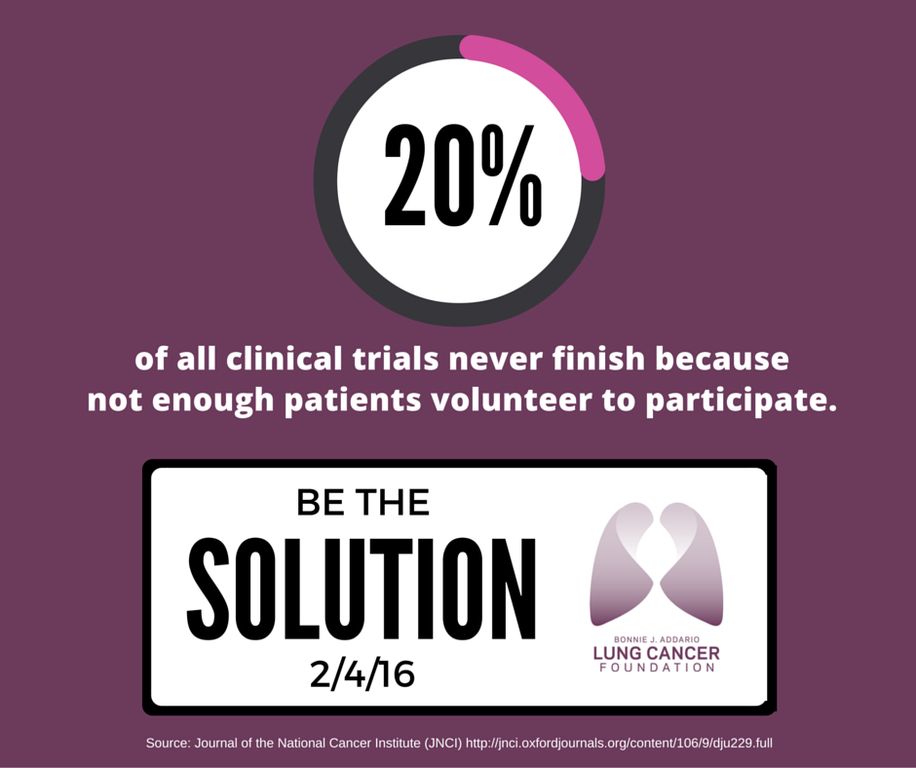

On average, 3-5% of all adult cancer patients participate in clinical trials!
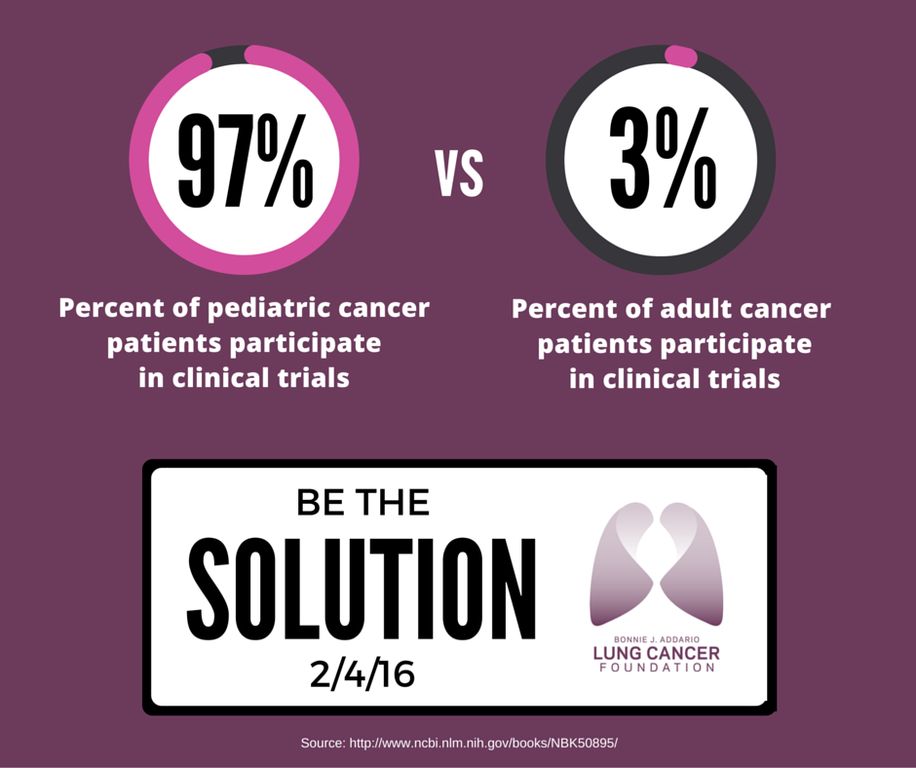
Participation is even lower among particular groups, including people who are racial and ethnic minorities, over 65, lower income, and living in rural areas. As an example, ~53% of new cancer diagnoses are in people 65 or older, however, this age demographic accounts for a measly 33% of clinical trial participants. Compare this to pediatric clinical trials!
So, what can we do to increase patient accrual to oncology clinical trials?
We need YOU to help us identify breakthroughs that will allow clinical trials to accrue patients quickly, so that we can get life-saving diagnostics and treatments to patients, faster!
Resgister now and submit your breakthrough solution to this problem! #BeTheSOLUTION!
Why an Innovation Prize/ Crowdsourcing Challenge?
We know that amazing ideas can come from anywhere! Despite 50 years of trying, members of the medical community have been unable to develop transformational solutions to these intractable problems.
So, we are trying something different! We want YOU to help us find cool ways to increase the accrual rate of cancer clinical trials!
We believe an Innovation Prize could have a profound impact on improving cancer care, too. Help us make it happen!
Register on this page and participate in this challenge to make an impact on the lives of cancer patients, and for the chance to win $150,000!
Contact Us:
- Have questions? Send us an email at, and we'll get back to you rightaway!
- If you have a solution that you believe could increase accrual rates but don’t know how to implement it, email us (). We will support you to determine how best to test the solution.
- We know the next breakthrough can come from anywhere and we are committed to finding a way to increase patient accrual to clinical trials!
The Challenge Breakthrough
The Clinical Trial Innovation Prize is looking for innovative means to double the number of cancer patients participating in clinical trials, from the current 3-5% for adult oncology clinical trials.
Increased participation in clinical trials will hopefully accelerate the pace of research and drug development allowing patients to live longer and better lives.
We are currently looking
- To identify prototypes of solutions that reduce a barrier to participation in clinical trials
- To select the best solution to be scaled in partnership with the Bonnie Addario Lung Cancer Foundation
If you have a solution that you believe could increase accrual rates but don’t know how to implement it, e-mail. The Addario Lung Cancer Foundation will support you to determine how best to test the solution.
Prizes
The Clinical Trial Innovation Prize will award a total of $180,000 in prizes
- Ideation Phase: $30,000 USD (already awarded)
- Implementation Phase: Two prizes worth $150,000 USD (to be awarded)
Our panel of judges will select first and second prize winners for the Implementation Phase, based on the judging criteria listed below.
- Implementation Phase First Prize: $100,000
- Implementation Phase Second Prize: $50,000
Submission Requirements
- Applications may be submitted either individually or by teams of innovators. There are no restrictions on age, country of origin, gender, educational accomplishments, etc. We are looking for brilliant, out-of-the-box thinkers and innovators to help us solve the problem of patient accrual to oncology clinical trials.
- The Implementation Phase of the Clinical Trials Innovation Prize has two rounds
- Proposal Round
- Proof-of-Concept Round
- All applicants who wish to participate in the $150,000 Implementation Phase of the Challenge need to submit a two-page proposal describing their solution, the specific barriers it addresses and how these will be overcome (details at https://herox.com/challenge/189). Top finalists from the Ideation phase will be automatically entered into the Implementation Phase, provided they provide an update on their proposals and any progress made since their previous submissions.
- All submissions will be evaluated by our panel of judges and a total of 6 applicants will proceed into the Proof-of-Concept round of the challenge. Top finalists from the first phase or Ideation phase of the challenge, and new applicants will be selected to enter the Proof-of-Concept round of the Clinical Trials Innovation Prize. New slots may be added if several promising applications are received.
- The Proof-of-Concept round of the Implementation Challenge will run for 6 months during which all finalist teams will be mentored by the Addario Lung Cancer Foundation, as well as our mentoring committees of key experts in the clinical trials space. ALCF will provide all finalist teams access to any resources they might need to test and implement their ideas to increase patient accrual to clinical trials.
- At the end of the 6 month implementation period, all challenge competitors should submit the following materials:
- Proof-of-concept materials
- Submit all relevant materials that demonstrate the solution (e.g., if creating an app or a website, provide a mock-up of the idea; or if suggesting an ad campaign, provide those materials).
- Impact measurement data analysis
- Competitors must provide evidence that their solution will have meaningful impact. This may include demonstrating that one or more relevant metrics (including, but not limited to, screening rates, accrual rates, refusal rates, changes in knowledge or behaviors, etc.) improve when testing the solution. They may reference peer-reviewed literature to support or interpret their data, if needed.
- Implementation write-up describing solution
- Questions in the submission form will guide competitors’ write-up describing and reflecting on their solution. Some questions will require a file upload.
Winning Criteria
Judges will evaluate submissions for the Implementation Challenge according to the following criteria:
PROPOSAL ROUND 1
|
Criteria
|
Description |
Score
|
| Solution Design |
How does the solution work? What does it look like? What does it take to create it? |
20 points |
| Innovation of Solution |
Creativeness of the proposed solution
|
20 points |
| Viability of Solution |
The probability that the solution will be created and implemented |
20 points |
|
Potential of sustainability & scalability of solution
|
The replicability of the solution and how it can be implemented across populations/locations
|
15 points |
| Implementation Plan |
The who, what, where and how of the solution |
10 points |
| Problem analysis and scope of solution |
Understanding of the barrier(s) and applying an appropriate solution to address them |
10 points |
| Cost |
Estimation of cost for the creation and implementation of the solution |
5 points |
| Total Points: |
|
100 |
PROOF OF CONCEPT ROUND 2
| Criteria |
Description |
Maximum Score |
| Impact |
- Effectively measures impact (choosing and tracking appropriate indicators)
- Features strong results that indicate an increase to accrual rate
- Impacts or has potential to impact a large audience
|
25 points |
| Solution Implementation |
- A strong solution that is well-implemented or prototyped
- High quality supplementary attachments displaying final product
|
25 points |
| Scalability and Sustainability |
- Show that the solution could be scaled to other health systems and target populations and sustain itself over time
|
25 points |
| Innovation |
- How the solution addresses a barrier in a novel or unique way
|
15 points |
| Cost |
- Benefit of the solution relative to the development and implementation costs
|
5 points |
| Analysis of Obstacles |
- Insight into challenges anticipated and encountered, with plausible solutions for how to address them to maximize impact
|
5 points |
| Total Points: |
|
100 |

















