From a quick internet search I found the following sites where a patient could register for a clinical study:
http://clinicalstudies.info.nih.gov/,
https://clinicaltrials.gov/ct2/home,
http://www.centerwatch.com/. From what I gather most of the sites allow studies to register and post their details. These sites are similar to a classifieds style posting, where emails/phone of the study organizers are listed.
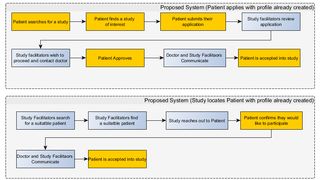
For a participant to register in the study, they must contact an email address or call the study facilitators directly to exchange information. It’s unspecified what type of information they must provide initially, so it’s bound to take a series of back and forth exchanges before the participant agrees to enter into the study and the study agrees to register the participant. Participants are able to contact studies based upon their review of the studies after locating via search, but the study facilitators have no means of contacting potential participants who are searching on the website, even if they have already provided information to another similar study.
In the current status quo, all of the effort is on the participant to locate the study, provide their information, and finally register in a study. With the small number of current participants, along with the high number of cancer patients who are unaware they can participate in clinical trials, it would be ideal if it was as simple as possible for participants to register in studies and have the study facilitators be able to perform most of the analysis of the potential patents to determine which are a good fit for their study.
People in general are increasingly accustomed to attaining products and services with the click of a button on their mobile device or computer. Amazon has reduced ordering a product to clicking a physical button in their home, which triggers an order with the Amazon Dash platform. Uber has reduced arranging a private car or taxi like service to opening an app and clicking a button to order a car. In both of these examples, no back and forth communication is required, the service or product is simply delivered at request with minimal effort by the consumer. This is the type of convenience people are used to today. If there is too much work required on the patients’ side, there is less of a chance they will register in a study.