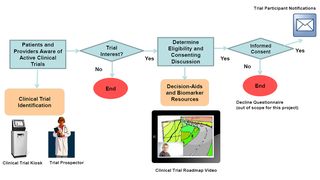
The landscape of clinical trials is changing; trial portfolios now include targeted, biomarker driven complex trials for lung cancer patients (eligibility based on existence/absence of specific tumor marker). Trial identification is increasingly becoming more complex. For example, patients often report uncertainty about delaying treatment while waiting the 4-6 weeks for biomarker results or waiting for slots to open on a specific trial. Many opt for standard treatment, due to fear of disease progression if treatment is not started immediately. Providers have a large portfolio of clinical trials available and need assistance identifying trial options based on patients’ individual medical history and current status. Current trial identification often requires communicating with multiple coordinators to determine eligibility.
Link 1: Physician recommendation is shown to be the most trusted source for introducing patients to clinical trials, as seen in this study where 73% of patients on clinical trials were recommended for the trial by their physician, yet only 10% of survivors were notified of available trials at their time of diagnosis. This may be a result of increased provider pressure to conduct research and see patients, coupled with increasing clinical trial complexity but by offering a segue/prompt into the clinical trial discussion at diagnosis, patients may be considered more often for a trial as a first-line treatment.
Link 2: This study suggests novel strategies to prompt and facilitate patient-provider communication regarding to clinical trial participation will lead to improved accrual rates. The study highlights the need for unique strategies for older and minority patient, who are approached less often with clinical trial opportunities.
Link 3: Patients are increasingly well informed and the potential exists for patients themselves to take a more active role in finding trials, assessing their eligibility and facilitating their enrollment.
Research advocates are integral in dissemination of research to the lung cancer community. Having advocates and the patient perspective incorporated in thoracic research during the planning stages is critical. Advocates can help identify potential barriers that could ultimately hinder accrual. The participation of past clinical trial patients in the content development and be share their stories in the roadmap video they will encourage prospective clinical trial participation. The video will highlight myths, potential barriers and how the patients overcame them. Overall, advocate involvement benefits research by ensuring that interventions intended for the patient population are relevant.
Our in-house data analyst monitors clinical trial accrual monthly. The detail reports distinguish between consented patients in the screening phase, on study, on treatment and off treatment. Baseline clinic volume and trial accrual data will be collected. Current accrual in our clinic is approximately 7-10% comparing new patients to those on trial. Despite past success, room for improvements exists and we continue to focus on interventions to improve minority accruals. We seek a 20% increase in accruals to therapeutic trials. If efficacious, the interventions could then be modified and adopted by other disease site clinics and outside institutions.
The campaign will be continuously evaluated. All products resulting from this campaign will be updated based upon the feedback obtained through post-intervention interviews/surveys. Based upon accrual rates, we will make improvements and supplement where needed.
We do not anticipate regulatory hurdles as we obtain IRB approval for the qualitative interviews with patients. We will recruit patients who meet outlined eligibility criteria, which, in addition to a lung cancer diagnosis are to: a) be > 18 years of age; b) have no documented or observable psychiatric or neurological disorders that would interfere with study participation (e.g., dementia, psychosis); c) be capable of speaking and reading standard English; d) primary diagnosis of lung cancer; e) completed initial visit in Thoracic Clinic (applies to new patients) f) participated in a thoracic clinical trial at MCC (applies to trial patients). There are no physical risks to the participant posed by this study. We will obtain informed consent and a media release for all stakeholders that participate in the development of the video.
For almost a decade, Moffitt’s Lung and Thoracic Tumor Education (LATTE) Program has focused on understanding the knowledge, attitudinal and system barriers to clinical trial participation and successfully developed intervention to address know barriers. Elements of our proposed solutions have been implemented before but what is unique and innovative about our proposal is that the outlined will be coordinated and cohesive. The elements will fall under the campaign aimed at improving clinical trial accrual in a complex clinical trial environment. While our efforts will be concentrated in lung cancer, we feel that if efficacious it can be easily replicated.